
Proper surface preparation is probably the most important factor affecting the total success of surface treatments for
corrosion protection.
New Technologies Combat Corrosion
With a vast selection of coatings and other protective products available in the
market and new, higher-tech solutions on the way, mine operators can choose
from many effective weapons to blunt the attack of a relentless chemical enemy
By Russell A. Carter, Managing Editor

The site-specific nature of corrosion problems and solutions may be the reason why estimates of the annual cost of corro-sion in the mining industry vary widely. The actual cost figure may not be critically important, but it’s high; a 2001 report from the U.S. Federal Highway Administration, for example, estimated that the overall annual cost of corrosion to critical sectors of U.S. infrastructure was more than $150 billion. More recently, a study by the National Association of Corrosion Engineers (NACE) pegs the total annual cost for organic and metallic protective coatings in the U.S. alone at $108.6 billion.
The NACE’s technical literature sug-gests that no material is resistant to all cor-rosive situations but materials selection is critical to preventing many types of fail-ures. Factors that influence materials selection are corrosion resistance in the environment, availability of design and test data, mechanical properties, cost, avail-ability, maintainability, compatibility with other system components, life expectancy, reliability and appearance.
Appropriate system design is also im-portant for effective corrosion control, and includes the consideration of many factors such as materials selection, process and construction parameters, geometry for drainage, avoidance or electrical separa-tion of dissimilar metals, avoiding or seal-ing of crevices, corrosion allowance, oper-ating lifetime, and maintenance and inspection requirements.
The bottom line: Experts believe 50% of all corrosion costs are preventable, and approximately 85% of these fall within the area of coatings. Industry vendors such as Sherwin Williams Protective & Marine Coatings, Akzo Nobel’s International Paint, Dulux Protective Coatings and others sup-ply a steady stream of new and improved anti-corrosion coating products each year, and many of these can be remarkably effective and long-lasting—if they are applied correctly, on a surface that has been properly prepared.
Test, Treat and Finish
Phoenix, Arizona, USA-based ChlorRid
International points out that protective coat-ings designed for steel, for example, perform
best when applied to a salt-free, clean sur-face. If the steel is coated before any one of
a number of harmful soluble salts is
removed from the surface, corrosion and
premature failure of the coating are likely.
Left unchecked, salt contamination cor-rodes deep into the substrate, making future
decontamination even more challenging.
For this reason, a number of industrial and
military organizations have established
tighter limits for acceptable soluble salt con-tent on surfaces prepared for coating.
ChlorRid has developed two salt testing kits: its ChlorIon Meter is a hand-held digital testing device that electronically measures chloride with an internal ion-specific elec-trode; ChlorTest is a complete system that the company said will allow even inexperi-enced inspectors to obtain accurate results. Once tested, surfaces can be cleaned by applying ChlorRid’s salt removal product.
Products and methods to test and remove salt contaminants are available from a variety of sources.
However, not every coating installation can take place under ideal conditions, and for that reason Sherwin-Williams added a new product to its Macropoxy family of high performance coatings—Macropoxy 80, a high-build HAPs-free epoxy formulat-ed for application over marginally prepared steel substrates and damp surfaces. The coating, according to the company, combats corrosion from both immersion and atmospheric exposures and can be applied at temperatures as low as 0°F (-18°C).
A modified phenalkamine epoxy, Macropoxy 80 is recommended for use on water and wastewater tanks as well as struc-tural steel, pipe, tanks and other equipment in industrial applications. Because of its surface tolerance, Macropoxy 80 can be applied in adverse conditions, and steel substrates need only to be cleaned of loose paint or rust (per SSPC SP2-3 Hand and Power Tool Cleaning) before application. Its high solids formulation (80%) reduces the likelihood of solvent entrapment that can lead to premature coating failure.

Peel-off, or Permanent
Not all coatings are made to last forever. For
example, Spraylat International, a U.K.-based company, offers the Protectapeel line
of environmentally friendly coatings that are
applied as a liquid by spray or roller and dry
to form a skin-tight, plastic film that can be
easily stripped away after use.
According to the company, Protectapeel is often ideal for protection of metal during shipping or storage. The product is claimed to prevent rust from forming on the pro-tected surface; when the items are ready to be installed or delivered the coating can simply be stripped off by hand, avoiding some common problems encountered with other protective products such as the need for solvent or hot-water cleaning to remove sticky residue, waxes or oils before the protected item can be used or installed. All Protectapeel coatings are water-based, making them safe for users and eliminating any need for special safety wear when working with the product.
Other coatings are designed to be prac-tically—or completely—invisible. For bear-ings and metal parts that are exposed directly to humid or corrosive environ-ments, NKE Austria GmbH offers a special galvanic coating that provides cost-effec-tive protection against corrosion. The new and improved version of its SQ171E coat-ing is thinner and provides longer-lasting protection against corrosion than the previ-ous version, according to the company.
SQ171E coating can be used for stan-dard or special bearings as well as for all metal parts exposed to wet or corrosive environments. According to NKE, even machined surfaces such as races can be coated, and the treatment provides protec-tion against water, condensation and slight-ly alkaline or acidic cleaning agents. Compared to uncoated components, parts coated with SQ171E are claimed to have a significantly longer service life. As an addi-tional option for even more effective protec-tion the coating is also available with a sili-cate-based sealing layer. Due to the reduced thickness of the coating (from 2 to 4 µm) coated and uncoated parts are completely interchangeable. When compared to stain-less steel, NKE says the SQ171E coating is more cost-effective, yet offers better anti-corrosion protection.

Meanwhile, according to results pub-lished recently from a joint university research project, a coating so thin it’s invisi-ble to the human eye has been shown to make copper nearly 100 times more resistant to corrosion, offering tremendous potential for metal protection even in harsh environments.
In a paper published in the September 2012 issue of Carbon, researchers from Monash University in Australia and Rice University in the United States said their findings could mean paradigm changes in the development of anti-corrosion coatings by using extremely thin graphene films. Graphene, a substance with atoms arranged in a regular hexagonal pattern similar to graphite, but in a one-atom-thick sheet, reportedly has a variety of potential applica-tions, including lightweight, thin, flexible yet durable display screens, electric circuits, and solar cells, as well as for enhancement of various medical, chemical and industrial processes. It also is attracting research attention for its possibilities as a means of increasing metal’s resistance to corrosion.
“We have obtained one of the best improvements that have been reported so far,” said study co-author Dr. Mainak Majumder. “At this point we are almost 100 times better than untreated copper.”
Dr. Parama Banerjee, who performed most of the experiments for the study, said graphene had excellent mechanical proper-ties and great strength. The polymer coat-ings often used on metals can be scratched, compromising their protective ability, but the invisible layer of graphene—although it changes neither the feel nor the appearance of the metal—is much harder to damage. “I call it a magic material,” Banerjee said.
The researchers applied the graphene to copper using a technique known as chemical vapor deposition, and tested it in saline water. “In nations like Australia, where we are surrounded by ocean, it is particularly significant that such an atomi-cally thin coating can provide protection in that environment,” Banerjee said. Initial experiments were confined to copper, but Banerjee said research was already under way on using the same technique with other metals.
Although the process is still in the lab-oratory-testing stage, Majumder said the group was not only looking at different met-als, but also investigating ways of applying the coating at lower temperatures, which would simplify production and enhance market potential.
While breakthroughs such as these are intriguing and offer the prospect of signifi-cantly enhanced corrosion protection for a variety of materials and equipment types, the most cost-effective solution for mining applications isn’t always the most high-tech method or product. On the following pages, author Joseph P. Langemeier offers a persuasive argument in favor of hot dip galvanizing—a process that was conceived and patented almost 200 years ago.
Hot Dip Galvanized Steel Reduces
Plant Maintenance Costs
By Joseph P. Langemeier
The maintenance of corrosion protection coatings on mining equipment is time con-suming, expensive and often neglected. This can lead to catastrophic equipment failures. Due to the triple protection pro-vided by hot dip galvanizing, coating main-tenance is virtually eliminated for the life of the equipment, which provides a safer working environment and defers mainte-nance expenses to other pressing needs.
The time spent repairing and replacing corroded steel items is time and money that could be better spent on other activi-ties. It’s frustrating for maintenance employees, expensive for mine owners, and very often causes shipping delays that upset customers. Hot dip galvanizing is a maintenance-free coating that protects steel from corrosion for many multiples of practical paint service lives.
The Galvanizing Process
To truly understand how hot dip galvanizing
saves lives, time and money, one first needs
to understand how and why it is so effective
at preventing corrosion on steel. Hot dip gal-vanizing is a thermo-chemical diffusion pro-cess that takes place in a vat of molten zinc.
As with painted steel, surface prepara-tion is key to a successful coating. If the steel is not clean, it will not galvanize. The metallurgical bond between zinc and steel is ensured by thoroughly cleaning the steel before immersing it in molten zinc. As part of the galvanizing process, steel goes through three cleaning steps:
• Degreasing—The first cleaning step, de-greasing, is often a hot alkali solution that removes organic contaminants like dirt, water-based paint, grease and/or oil. After degreasing, the article goes through a water rinse. Any epoxies, vinyls or asphalt coatings must be removed by mechanical means (grit blasting, etc.) before steel is taken to the galvanizer.
• Pickling—Next the steel is moved to the pickle bath, an acidic solution of either ambient hydrochloric acid or heated sul-phuric acid, that removes iron oxides and mill scale from the surface of the steel. After pickling, the steel is rinsed again.
• Fluxing—Finally, the steel moves into the flux tank. The flux serves two purposes. First, the lightly acidic solution cleans any remaining iron oxides; and second, it provides a protective layer to prevent any iron oxide formation prior to immersion in the galvanizing kettle.
The true “galvanizing” phase of the process consists of completely immersing the steel in a minimum 98% pure zinc bath. The bath temperature is maintained at 815°F (435°C) or higher. The steel is lowered at an angle by crane hoist. This allows air to escape from tubular shapes or pockets that may be within the design of a fabricated piece and permits the molten zinc to displace the air. Approximately 5 to 7 minutes after complete immersion (de-pending on the size of the articles), the steel reaches the bath temperature and the metallurgical reaction is complete.
The last phase of the process is the final inspection. A very accurate determination as to the quality of the galvanized coating can be accomplished through a visual inspection of the material. As stated earli-er, if the steel surface is not properly and thoroughly cleaned, the zinc will not adhere to the steel. Additionally, a magnetic thick-ness gauge can be used to determine the thickness of the coating to ensure compli-ance with specification requirements.
Coating Structure
and Performance
During the galvanizing process, the zinc in
the kettle metallurgically reacts with the
iron in steel to form a series of zinc-iron
(intermetallic) alloy layers. The first zinc-iron alloy layer, the Gamma layer, is
approximately 75% zinc and 25% iron.
The next layer, the Delta layer, is approxi-mately 90% zinc and 10% iron. The third
layer, the Zeta layer, is approximately 94%
zinc and 6% iron. The last layer (Eta),
which forms as the material is withdrawn
from the zinc bath, is identical to the zinc
bath chemistry, i.e. pure zinc. The Gamma,
Delta and Zeta layers form approximately
60% of the total galvanized coating, with
the Eta layer making up the balance.
Because the galvanizing process in-volves total immersion of the material into cleaning solutions and molten zinc, all interior and exterior surfaces are coated. This includes the insides of hollow and tubular structures, and the threads of fas-teners. Complete coverage is important because corrosion tends to occur at an increased rate on the inside of some hol-low structures where the environment can be extremely humid and condensation occurs. Hollow structures that are painted have no corrosion protection on the inside. Additionally, fasteners with no protection on the threads are susceptible to corro-sion, and corroded fasteners can lead to concerns about the integrity of structural connections.
Three elements (barrier protection, cathodic protection and the zinc patina) are what provide galvanizing its long-last-ing protection. Similar to paints, the hot dip galvanized coating provides barrier pro-tection from corrosion. The zinc coating acts as a barrier against the penetration of water, oxygen and atmospheric pollutants, as well as electrolytes in the environment. As long as the barrier is intact, the steel will be protected and corrosion will not occur. However, if the barrier is breached, corrosion will begin. The impervious nature of zinc makes it a very good barrier coating. Aged zinc corrodes approximately 1/10 to 1/40 the rate of steel depending on the environment, making the corrosion rate of a thin zinc coating equivalent to a much thicker steel piece.
In addition to barrier protection, galva-nizing also provides cathodic protection to steel, which means zinc will preferentially corrode to protect the underlying base steel. The zinc coating cathodically pro-tects the steel from coating imperfections caused by accidental abrasion, cutting, drilling or bending.
Zinc coatings “age” with time and expo-sure to the atmosphere. This aging results in a tightly bonded, thin layer of zinc car-bonate on the surface that is often referred to as the “zinc patina.” This patina is impervious, and passive, which slows the corrosion rate of the zinc. The zinc patina, which is a critical part of coating’s longevi-ty, requires natural wet and dry cycles to form. Accelerated tests (salt spray) do not accurately predict the life of a galvanized coating because they are conducted under a constant salt fog, inhibiting the formation of the passive zinc patina.
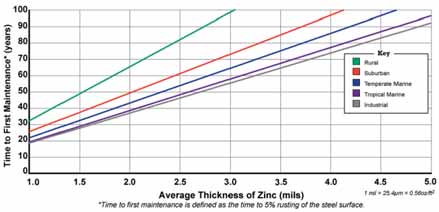
Using a Time to First Maintenance Chart (See Figure 1), developed using the Zinc Coating Life Predictor Model by Dr. Gregory Zhang of Teck Cominco, the galva-nized coating’s protective life is shown within the five atmospheric environments defined by ASTM International. A galva-nized coating’s protective life is deter-mined primarily by the thickness of the coating and the severity of the exposure conditions. The thickness of a galvanized coating is expressed in “mils.” A ‘mil’ is equal to 1/1,00 th of an inch.
According to ASTM A1234, a piece of steel 1/4 inch thick or greater must have a minimum of 3.9 mils of zinc. More often than not, you will get greater than the min-imum requirement when hot dip galvaniz-ing, so for ease of using the chart, would equate to 4 mils. Backtracking the 4 mils up to the industrial (gray) environment line and across, the piece will have approxi-mately 72 years until first maintenance (or 5% rust of the substrate steel). In other words, 95% of the coating is still intact, so the structural integrity is not threatened. It is merely time to apply a corrosion-resistant coating on the structure to extend its life.
Cost Comparisons
A common misconception is that galvaniz-ing is cost prohibitive. When specifying
steel corrosion protection systems, it is
important to consider not only the initial
cost, but also the life-cycle costs (includ-ing all future maintenance). When life
cycle costs are considered, hot dip galva-nizing is the most economical system for
corrosion protection.
Even if you think designing equipment members to fit into the galvanizing kettle may mean more erection time in the field and slightly more steel and connections, (by the way, 60-ft kettles are now common) those costs are generally small when com-pared to the savings indicated. When the hidden (indirect) costs (estimated to be five to 11 times the direct costs) associat-ed with the maintenance of paint systems is added to the analysis, galvanizing is clearly the most economical choice.
Joseph Langemeier (joelanemeaier@azz.com) is marketing manager for AZZ Galvanizing Services, headquartered in Ft. Worth, Texas, USA.