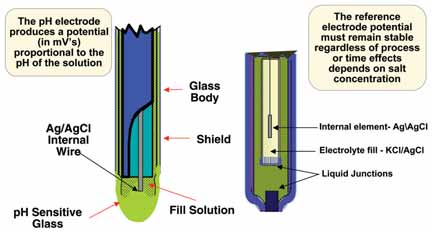
Figure 1: Conventional pH sensor.
Improving Productivity and Safety Through More Efficient Separation and Leaching
Advanced diagnostics are the key to reliable pH and ORP measurements
By Dave Joseph

When the pH and oxidation reduction potential (ORP) values in process fluids are correctly maintained based on accurate instrumentation, the release of cyanide gas is prevented, and the destruction of dissolved cyanide in wastewater is assured. When operators can count on the validity of every measurement, they don’t feel the need to apply extra chlorine to assure cyanide removal, saving money and avoiding potential problems with excess chlorine in effluent streams. In addition, the continuous record of online measurements taken on effluent streams can be most useful in providing proof of compliance if required by regulators.
However, accurate and reliable measurements are not always easy to obtain in separation and leaching operations because the sensors are regularly subjected to coating and/or breakage when immersed in harsh mixtures. In some cases, they may not even be immersed. Measurement accuracy is greatly increased by using advanced, microprocessor- based instruments with state-of-the-art sensors designed to withstand the rigors of these processes. Reliability is assured through diagnostics that inform operators if a sensor is coated or broken and needs to be cleaned or replaced.
The real value of monitoring pH and
ORP in metals processing is realized when
mine personnel are able to rely fully on
their instruments for better process control,
resulting in:
• Maximum yield/throughput;
• Reduced separation costs with minimum
waste;
• Stable process operations;
• Better protection of personnel and the
environment; and
• Lower labor and maintenance expenses
based on latest calibration techniques
and good training.

Flotation is the most common method of concentrating crushed copper ore, whereby iron and other contaminants are separated from the copper sulfide slurry. In this process, copper sulfide particles latch onto bubbles in a froth created by foaming agents and air injected into the flotation cells. The copper-rich froth is skimmed off and dried to form a copper concentrate. The effectiveness of this concentrating step depends on maintaining a specific pH range so the bonding action can take place. With the recent drop in copper prices, a more efficient concentration process may be the key to continued profitability.
A well-regulated flow of lime slurry is used to keep the pH in the flotation cells within an acceptable range. If the pH is too high, iron will be entrapped with the copper, decreasing the value of the copper concentrate. If too much lime is added, the result is a diluted froth that requires further concentration in subsequent stages, wasting lime and increasing operating costs. This is why accurate and reliable pH measurements are so necessary.
Both accuracy and reliability are likely to be altered if the pH sensor becomes coated, which is a very real possibility when immersed in the frothy liquid. A “best practice” is to use sensors specifically designed to resist coating. Also, the analyzers mounted near the flotation cells should transmit continuous diagnostics to alert plant personnel if a sensor has become coated, causing erroneous signals. The operators will then know the readings are unreliable, and the sensor should be cleaned.
In the leaching and extraction of precious metals where cyanide is commonly used, both pH and ORP measurements are essential for efficiency and safety of personnel. For example, pH values below 11 may allow the formation of hydrogen cyanide (HCN) which is a highly toxic, yet colorless gas. Since the formation of HCN removes active cyanide from the leaching process, effective pH control both improves the leaching process and minimizes the risks to the environment and mine personnel.
In waste treatment, it is very important for personnel to know both the ORP as well as the pH to control and verify the total destruction of waste cyanide streams. An oxidant, typically chlorine, is commonly used to prevent cyanide from entering waste streams, and the ORP indicates whether the solution is under oxidizing (high ORP) or reducing (low ORP) conditions. Since cyanide cannot be present under oxidizing conditions, an oxidant is added to the appropriate stream, causing the ORP to rise and the cyanide to be converted to cyanate and then to harmless nitrogen and carbon dioxide.
By carefully watching the pH and ORP readings, mine personnel are able to maintain a proper balance between the amount of chlorine used and cyanide destruction. The reaction is complicated but is best managed when mine personnel receive accurate information from dependable instrumentation. Having accurate pH improves the accuracy of ORP readings by removing interference from other chemicals. In addition, maintaining the correct pH is necessary to protect against a discharge of hydrogen cyanide gas which can be generated during the multi-stage process by the addition of too much chlorine.
Sensor Selection
Conventional pH sensors have a measurement
(typically glass) electrode and a reference
electrode to complete a current
loop (See Figure 1). Both must be in contact
with the process solution, but any
coating build-up around either electrode
will degrade performance, slow the
response, and eventually render the sensor
unusable.
The glass membrane is very thin and can be broken if hit with a sharp or hard object. Thicker glass is more resistant to impact damage, but more likely to be damaged by temperature swings. Interestingly, fewer pH sensors are damaged by impact than one might think. The hemispherical dome of the glass bulb is actually quite strong. The reference electrode uses a small flow of salt solution (electrolyte) to complete the current loop through the process. If the reference is blocked by coating, the current is disrupted and the pH sensor is no longer functional.

Visual inspection rarely reveals faulty sensors. They can be checked in a buffer where a slow responding sensor can be identified. However, this takes time, and mine personnel rarely remove sensors to check their condition.
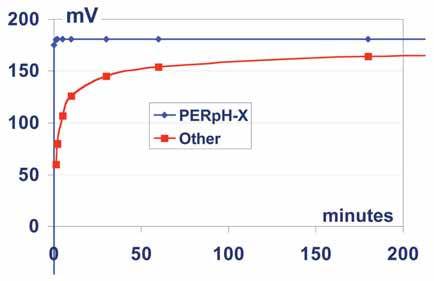
State-of-the-art sensor technology overcomes these issues by delivering longer life sensors that respond very quickly to process changes and need cleaning less frequently. This is very important in protecting both the environment and mine personnel from possible exposure to the dangerous effects of the chemicals involved, and the Rosemount PERpH-X sensor is designed for just such service (See Figure 3). Both pH and ORP measurements are made with similar sensor designs, differing only in measurement electrode.
New analytical devices generate online diagnostics, providing additional value by informing users when a sensor is not immersed, needs cleaning, or is broken and needs to be replaced. They can even tell if sensor performance is below expectations.
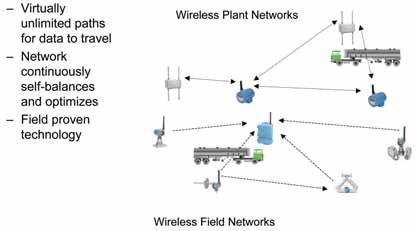
When these analytical devices are used as components of Emerson’s PlantWeb plant automation architecture and DeltaV control system, the field-generated diagnostics can be used for instrument reconfiguration and calibration without sending technicians out to the analyzers themselves. The Rosemount Analytical solution keeps mine personnel informed of the status of their sensors and whether they can be relied upon to deliver accurate readings.
Wireless technologies can have a dramatic impact on mining operations in two broad categories: wireless field networks for sensor and field device applications, and wireless plant networks for business and operations purposes (See Figure 4).
Self-organizing wireless field networks (WFN) are based on the WirelessHART communications standard which was adopted in 2007 by more than 200 member companies of the HART Communication Foundation.
The field devices in a self-organizing wireless mesh network actually communicate with each other, so there is no single point of failure. Every device serves as a network connector, automatically finding the optimum communication pathway to the network gateway. In the event a temporary obstruction blocks a direct connection, signals are automatically rerouted via an adjacent device, ensuring network reliability and data integrity.
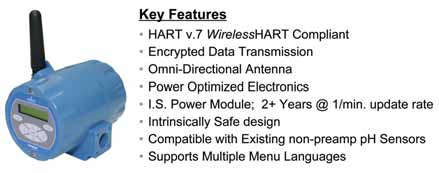
Numerous WirelessHART field devices are available for a wide range of applications. Figure 5 is one example. Others are designed to transmit temperatures, pressures and flows; data from vibration monitors and analytical equipment; and valve position and deviation to process control and asset management systems.
Wireless devices can be installed quickly and easily where wired devices cannot— in hard to reach locations, areas hazardous to mine personnel, where power doesn’t exist, and where running wires is not allowed, to name a few. Data retrieved from these devices enables mineral processors to respond to equipment problems immediately and even predict when problems will occur in order to make timely repairs and avoid unexpected downtime. With this will come process improvements and greater equipment availability. The ultimate benefit is a combination of lower costs and increased productivity.
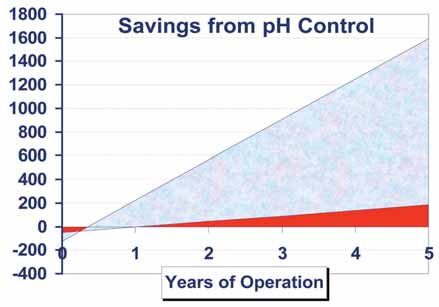
Mines can save money by utilizing continuous online pH and ORP measurements along with the diagnostics available from today’s smart analytical instruments. A return on investment in advanced technology can be achieved within one year (See Figure 6).
Dave Joseph is senior industry manager for Emerson Process Management.