Atlas Copco’s Weda pump line.
Solvent Extraction Solutions
Rethinking Copper Recovery
At the latest of Alan Taylor’s hydrometallurgical
technology conferences, ALTA 2008
Nickel-Cobalt, Copper & Uranium, held in
June 2008, Bateman Engineering staff
presented four papers in all covering uranium,
cobalt and copper recovery. One of
these looked at three options for the leaching
and solvent extraction of copper from
oxide ores and another evaluated the applicability
of two other such process routes to
the treatment of mixed secondary and primary
copper sulphide deposits.
Oxide Copper Issues
Bateman Engineering and Cognis specialists
in Australia and North America have
evaluated a new flowsheet proposed by
Cognis. This design is intended to maximize
net revenue largely through reductions
in acid make-up. The evaluation compared
the new conceptual but technically
feasible design with two other leach—
solid/liquid separation—solvent extraction
circuits that are in commercial operation:
the circuit used at the pioneering Nchanga
Tailings Leach Plant in Zambia and the
Split Circuit configuration. The study
assumed that 80% of the recoverable copper
is leached in the first leaching stage.
The driver for this bid to minimize the adverse financial impact of high leach acid costs on copper hydrometallurgical project viability is the significant increase in acid costs that has resulted from the pressure on supply in the Central African Copperbelt. This is in turn due to the success of copper oxide leaching operations on the one hand and the high gangue acid consumption involved on the other.
Metsim Modeling
The evaluation utilized Metsim modeling to
compare the three plant configurations,
each intended to treat an oxide/secondary
sulphide deposit treating a 4% copper
head grade and a 0.05% cobalt head grade
at a treatment rate of 2.5 million mt/y.
This will provide a copper feed tonnage of
100,000 mt/y and 1,325 mt/y of cobalt.

The Metsim Models used in this analysis were derived from an engineering model developed as part of the engineering design for an oxide leach circuit. The base model was modified as required to develop the three flowsheets, keeping the basic design parameters of the unit operations unchanged across the three options.
The model was inclusive of all of the design and engineering detail required for detailed design so the derived models provide a very comprehensive basis on which to compare the performance of the three flowsheets. Each flowsheet was run with two wash ratios for the counter current decantation (CCD) circuit in each case to investigate the impact of a higher wash ratio on cobalt recovery and the size of the iron removal and cobalt precipitation circuit.
A number of iterations of each circuit were performed using Cognis Isocalc software to determine the copper extractions for each SX circuit in order to refine the copper extraction performance.
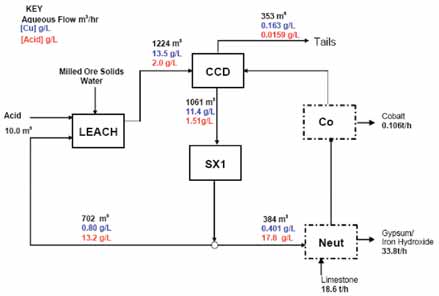
In the study Flowsheet 1, the Conventional Circuit is a simplified version of the metallurgical flowsheet in use for many years at the Tailings Leach Plant at Nchanga, Zambia, amended to include cobalt recovery. This base case comprises a single train circuit using an atmospheric leach circuit, followed by a CCD train with solvent extraction on the CCD 1 overflow. A bleed stream of copper raffinate is neutralized to remove acid and copper and the cobalt is recovered by precipitation. The SX circuit comprises three extraction stages and two stripping stages.
The raffinate bleed is treated through a two-stage iron removal circuit, with the first stage removing the majority of the acid, iron and manganese. The precipitate from the second stage removes the residual copper, iron and acid is recycled to atmospheric leach. Cobalt is recovered by precipitation. The acid and sulphate loading in the feed to iron removal is the highest of all of the options. Higher entrained cobalt losses were identified as a result of the higher gypsum and iron hydroxide production.
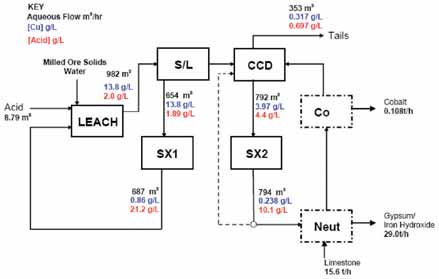
A bleed of SX2 raffinate is treated though an iron removal circuit and the cobalt is again recovered by precipitation. The acid and sulphate loading on the iron removal circuit is substantially lower than the base case, which minimizes acid loss from the overall circuit and with it the neutralization costs and cobalt entrainment losses.
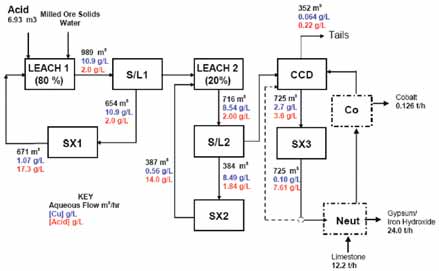
Flowsheet 3, the Sequential Flowsheet, is a new flowsheet proposed by Cognis and is based on a split leaching concept to maximize retention of acid in the leach circuit, thus minimizing acid loss to the cobalt circuit.
The circuit configuration comprises two leach trains in series, with an intermediate solid/liquid separation stage from which the first SX circuit derives its feed. SX1 raffinate is recycled to the head of the leach. A second solid liquid separation stage follows the second leach train which again operates in closed circuit with a second SX circuit, with raffinate recycled back to the head of the second leach.
Underflow discharge from the second solid/liquid separation is washed in a CCD circuit and a final stage of solvent extraction is used to recover the remaining copper. A bleed of third stage raffinate passes to cobalt recovery. Again the acid and sulphate loading on the iron removal circuit is substantially lower than the base case minimizing acid loss from the overall circuit and, consequently, neutralization costs and cobalt entrainment losses—but to an extent that exceeds that of Flowsheet 2.
Results Assessment
Bateman and Cognis have presented[1] the
Metsim modeling results as: revenue projections
based on then current and also
long-term metal pricing; operating cost differentials
relative to the base case; capital
cost differentials relative to the base case;
and net revenue projections.
In order to achieve worthwhile revenue and operating cost savings it was necessary to alter some of the equipment sizes and add some units to the base flowsheets, including an additional thickener, one or two pinned bed clarifiers, extra SX trains and ponds, and scaled up cobalt recovery circuits. But, despite the differences in these flowsheets, both copper and cobalt recoveries are generally similar. And, while the multiple SX trains considered do increase circuit complexity, they reduce the potential impact of fire breaking out in any one train.
The two companies consider that this kind of evaluation can be used as a tool for minimizing operating costs, particularly the level of consumables required—which, they point out, is a significant issue for remote plant sites with extended logistic support. The modeling indicated that the Cognis flowsheet reduces the sulphate mass flow to the bleed circuit by reducing the copper concentration in that stream. Minimizing the copper concentration in the pregnant leach solution supplied to the SX circuit preceding the bleed stream contributes to a reduction of the neutralization costs for the bleed stream and the concurrent cobalt wash and co-precipitation losses. This flowsheet delivers the best net revenue projections and offers potential for substantial operating cost savings.
Mixed Secondary and
Primary Sulphide Deposits
In the other copper presentation[2] Bateman
Engineering Perth staff turned their
attention to copper sulphide deposits that
require first the treatment of secondary sulphide
ore, followed by a transition through
an increasing proportion of primary sulphide
ore to entirely primary material. In
this situation it is preferable that the
process plant can handle the variation cost
effectively. But in practice treating the primary
sulphide ore component often seems
to result in higher operating costs and/or
lower metal recovery. Or a major capital
investment may be required to modify the
original flowsheet.
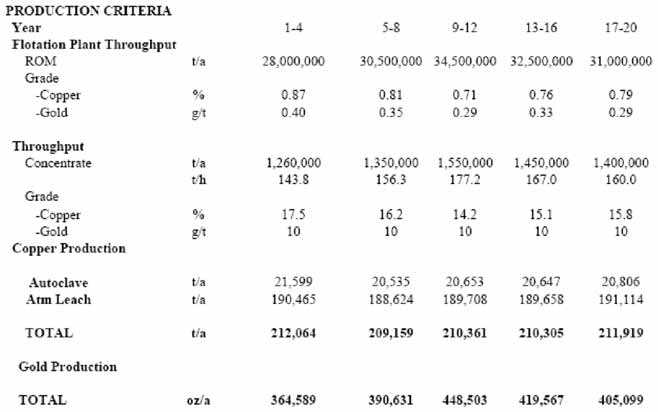
Bateman examined two process options that may be able efficiently to recover copper and any associated precious metal values throughout the secondary to primary sulphides treatment transition with no significant added capital cost or increases in operational costs. The team compared the Galvanox process with the Sepon Copper process (incorporating a proposed small modification) for treating a hypothetical copper-gold deposit with a mixture of secondary and primary sulphide ores. The plants were designed to yield 200,000 mt/y copper cathode with an average gold output of 400,000 oz/y (Table 1).
The Galvanox and Sepon processes are both able to treat copper concentrates that contain significant quantities of arsenic. The copper leach releases the arsenic into solution. The trivalent arsenic leached into solution can then be oxidized to the pentavalent state and precipitated as ferric arsenate, FeAsO4 (scorodite), through an oxyhydrolysis steps. Scorodite is a stable arsenic compound able to be safely discharged in a lined tailings impoundment.
Galvanox Process
The Galvanox process is patented technology
developed by the University of British
Columbia that takes advantage of the galvanic
couple between pyrite and chalcopyrite
to ensure rapid and complete oxidation
of chalcopyrite under atmospheric, medium
temperature conditions in acidic iron
sulphate solution, without the need for
microbes, ultrafine grinding, or chemical
additives such as chloride, nitrate, or surfactants.
Other copper sulphides are also
leached in parallel, probably by a similar
mechanism but generally with faster leach
kinetics. Arsenic bearing copper sulphides
give somewhat longer leach times.
Copper recoveries of 98% or greater have been achieved under these conditions in as little as four hours residence time, but more typically about 20 hours depending largely on the extent of pyrite recycle. The process is selective for chalcopyrite over pyrite, with the pyrite passivated and unleached. It generates near quantitative levels of elemental sulphur, and is fully compatible with conventional solvent extraction and electrowinning of LME Grade A copper cathodes. A high pressure autoclave is used to treat approximately 10% of the concentrate to provide a source of acid and heat for the atmospheric leach and to precipitate iron leached from chalcopyrite. The Galvanox process effectively leaches primary copper sulphides in the atmospheric leach in a short residence time by maintaining close control over the atmospheric leaching parameters, in particular electrochemical potential.
Locating a hydrometallurgical facility that yields final metal product in the form of copper cathodes at the mine site eliminates costs associated with drying and transporting the concentrate to a smelter–refinery, plus treatment charges (TCRCs). Using the Galvanox process to treat concentrates at a site also eliminates penalty charges for impurities such as arsenic. Galvanox can be applied to the entire concentrate output, particularly where dirty concentrates create smelting issues. However, smelter TCRC costs are currently at close to historical lows, which reduces the competitive advantage of any hydrometallurgical treatment route for concentrate treatment as less net revenue is available to cover the capital expenditure of the plant.
An alternative flowsheet option utilizing the Galvanox technology is available. It minimizes this latter impact by providing a revenue stream which is largely independent of smelter charges, being based on copper in the cleaner tailing not normally recovered to final concentrate. The concept requires a somewhat different approach to the operation of a flotation concentrator, using Galvanox to treat only the cleaner tailings stream, not the entire high grade concentrate stream. The approach takes advantage of the fact that pyrite is not oxidized in a Galvanox circuit, allowing copper streams as low in grade as 1% copper to be treated cost effectively.
In a flotation concentrator, maximum grade and maximum recovery are mutually exclusive targets and typically result in a 2%-4% copper loss between the roughing and cleaning stages. It is this copper loss that the proposed flowsheet targets, together with any additional copper recov- Table 1 - Production Criteria. ery losses attendant on maximizing concentrate grade. Substantial reductions in freight and smelting costs for concentrate can be achieved by this approach and the net savings generated are independent of smelter costs.
So far, Galvanox has been considered for high grade concentrate streams but the process is very flexible and a range of treatment options are available if the capabilities of this technology are fully utilized. A process plant based on treating cleaner tailings is smaller, does not place the entire concentrate revenue at risk from the application of new technology, and generates a copper revenue stream currently lost to tailings.
Sepon Copper Process
The Sepon Copper Process is an atmospheric
and high-pressure leach process
which can efficiently treat secondary copper
sulphides via a ferric-assisted atmospheric
leach. It has been commercialized
successfully for a whole of ore leach at the
Sepon Plant in Laos.
The “standard” Sepon Copper process uses ferric-assisted atmospheric leaching for secondary copper sulphides. The atmospheric leach residue is then floated to recover pyrite, which is treated via total pressure oxidation through a high temperature and pressure autoclave to create the ferric and acid required for the atmospheric leach. It also creates heat to maintain the atmospheric leach at the required temperature.
Modifying the flowsheet and floating the chalcopyrite with the pyrite allows the copper to be recovered through the total pressure oxidation process, a commercially proven method for treating primary copper sulphide ores, whilst still generating heat and acid used in the atmospheric recovery of the secondary sulphides. Like the Galvanox process, the Sepon process locks arsenic into an environmentally stable form, which allows the treatment of arsenic contaminated concentrates.
Gold is recovered from both of these flowsheets from the leach tails in a traditional CIP/CIL circuit. Gold recovery from both processes has been shown shown to be greater than 90% in batch testing.
Primary and Secondary Sulphides Treatment
Both of the processes use an autoclave,
but only to process a small portion of the
ore, not the entire ore or concentrate. This,
in itself, is a major step forward, allowing
unadulterated primary copper sulphide
ores to be treated without the high capital
and operating costs of a total pressure oxidation
system.
The Galvanox process allows the primary sulphides to be leached primarily in an atmospheric leach at 80°C. This is achieved by treating approximately 10% of the concentrate in a high temperature autoclave, which provides the reagents required for the atmospheric leach. The addition of the pyrite recycle from the underflow of the solid/liquid separation circuit to the atmospheric leach provides the catalytic effect for the leaching of chalcopyrite without passivation. The pyrite itself does not leach, which is a critical part of the Galvanox leach. Galvanox leach conditions preferentially leach chalcopyrite so the pyrite is available for use as the catalyst post leach, and does not create heat or acid which would require neutralization with associated costs.
In the Sepon process, the primary sulphides are recovered after the secondary copper sulphides are leached in the atmospheric leach. The primary sulphides, pyrite and gangue are separated from the solution in the solid/liquid separation. The primary copper sulphides are then separated from the pyrite and gangue via flotation to produce a concentrate rich in primary copper sulphides. This concentrate is then treated via total pressure oxidation in a high temperature autoclave as in a traditional total pressure oxidation. But the preceding unit operations recover all the other copper and remove pyrite and gangue materials, greatly reducing the autoclave capital and operating costs due to the smaller size of and flow through the autoclave. The Sepon process also uses the heat, acid and ferric produced in the autoclave in the atmospheric leach.
Capital and Operating Costs
The main equipment difference between
the two processes is the addition of a flotation
circuit to the Sepon process. However,
all the equipment will be sized slightly differently
for each process. The overall estimated
capital cost in US$, excluding contingency
and based on costs prevailing in
third quarter 2007 equate to $3,450/mt
copper for the Galvanox process and
$3,500/mt copper for the Sepon option,
albeit with a +/-30% accuracy resulting
from the limited data availability.
It is possible to change the function of the plant from a Sepon process to a Galvanox process. This would require rerouting of pipework to set the plant up as a Galvanox plant, but the third autoclave train would not need to be installed. If this method is chosen, the two autoclaves to be installed initially should be the slightly larger size autoclaves (4.85-m diameter). There would be some new pumps, valves and instrumentation required. The capital is considered to be minimal but there would be some plant shutdown time while the switch was being made.
Operating costs were determined for the major variable operational cost centres for the two options. The assumed cost of power was $5.5c/kWh. For Galvanox the total operating cost per pound of copper was $0.138 and for Sepon $0.154.
Conclusions
Both the Sepon and Galvanox processes
are suitable for treating large, low grade
copper sulphide deposits to effectively
recover copper and gold and lock arsenic
into an environmentally stable form.
The Sepon process has the flexibility to process a range of copper sulphide mineralogies, but it requires alterations to the circuit as the level of primary sulphides increases. The Galvanox process is able to treat both primary and secondary copper sulphide mineralogies without flowsheet modifications.
As the proportion of primary copper sulphides increases over the life of the mine, the operational costs of the Sepon process will increase due to the mechanism by which the Sepon process recovers copper from the primary copper sulphides. This is not true of the Galvanox process, where the operational costs remain reasonably constant over the life of the mine, independent of the mineralogy of the entrained copper.
The long term capital costs for both the processes are very similar, and within the accuracy of the study. The similar processing capabilities and costs suggest both processes offer operators robust, viable solutions for treating copper sulphides.
References
1. Baxter K., A Nesbitt, M Urbani and K
Marte: Flowsheet alternatives for high
acid consuming copper ores.
2. Miller K J, L A Sylwestrzak and K G
Baxter: Treatment of copper sulphide
deposits – evaluation of a Glavanox™
versus Sepon circuit configuration.
Both papers presented to ALTA 2008
Nickel-Cobalt, Copper & Uranium conference,
Perth, Australia, June 2008.